Titania – the White Fairy in your Home
Titania was the fairy queen in Shakespeare’s A Midsummer Night’s Dream.

It is also the name of titanium oxide TiO2. (Like magnesia for magnesium oxide and others.) Though discovered in 1821 it was only in 1916 that chemical engineers were able to produce it pure on a large scale for commercial use.
It gives a brilliant white for paint and paper, and by replacing lead in household paint enormously reduced lead poisoning of industrial workers and children in the home.
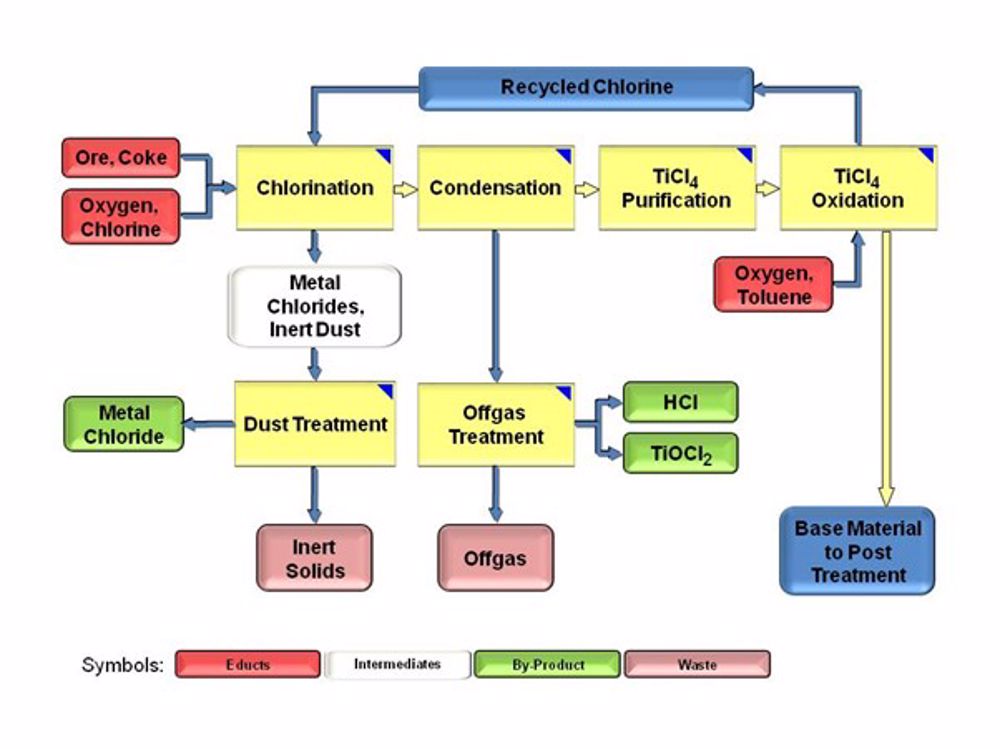
This brilliant white often comes from the mineral known as ‘Black Sand’ by a challenging process in which it is reacted with elemental chlorine Cl2 to give titanium chloride, TiCl4 which is a vapour at the reaction temperature (and sometimes referred to as ‘tickle’). This is separated from the dirt in the ore, condensed and purified by distillation. It is then reacted with oxygen O2 to give pure TiO2 and chlorine. The chlorine is recycled, and the solid TiO2 is ground down to the size required and treated for its end use.
TiO2 (crude) + 2Cl2 + C = TiCl4 + CO2
TiCl4 + O2 = TiO2 + 2Cl2
Note that though the starting material is a fairly inert rock, and the product is a clean non-toxic powder, the intermediates are the highly toxic and corrosive, and the process operates at high temperature. It is the job of chemical engineers to design and manage such processes safely.
There are a number of complications to give a pure product and deal with waste effectively.
The white pigment is not only used in paint and paper, but in plastics, sunscreens, cosmetics and other products. The particle shape and size are controlled for the use: a whole extra chemical engineering process, including other synthetic routes. It is also used in electronics and optics and has promise for a new generation of batteries.
A recent development is use as a photocatalyst, so with UV or sunlight a suitable surface can destroy organic pollutants in wastewater, or, on buildings, in the air.
TiO2 is non-toxic so is added to toothpaste and some foodstuffs, number E171 in Europe. [However, the European Food Standards Agency has recently (May 2021) decided that it cannot rule out genotoxicity. That is, it may contribute to DNA damage. While DNA is damaged many times a day (and repaired) in our cells, severe damage can lead to cancer. There is no evidence that it does give an increased cancer risk, but its use in food is likely to be withdrawn or reduced.]
Titania is in most paints (except for very dark colours) and a host of objects about the house. Whiter than snow, it only needs to be used in tiny amounts in surface coatings, but some 6 million tonnes of TiO2 are produced each year by the above and some older processes, with a value of about £12 billion. In a light bright modern home, Titania is all around.