The Story of Plastic: Past, Present, Future
Plastics are polymers (long-chain molecules) or combinations of such molecules with other substances to give the desired properties. In the 19th century these were all based on chemical treatment of natural polymeric substances such as rubber and cellulose.
The twentieth century has been the time when modern technological society came about, and plastics have been at the heart of this development. Few modern necessities would be possible without them. They have displaced traditional materials, but also created new technology which would be impossible without them. However, a laboratory discovery is not enough. To make them on a large scale is itself a major task, and it is making them cheap enough which has brought them from expensive specialist objects to widespread use.
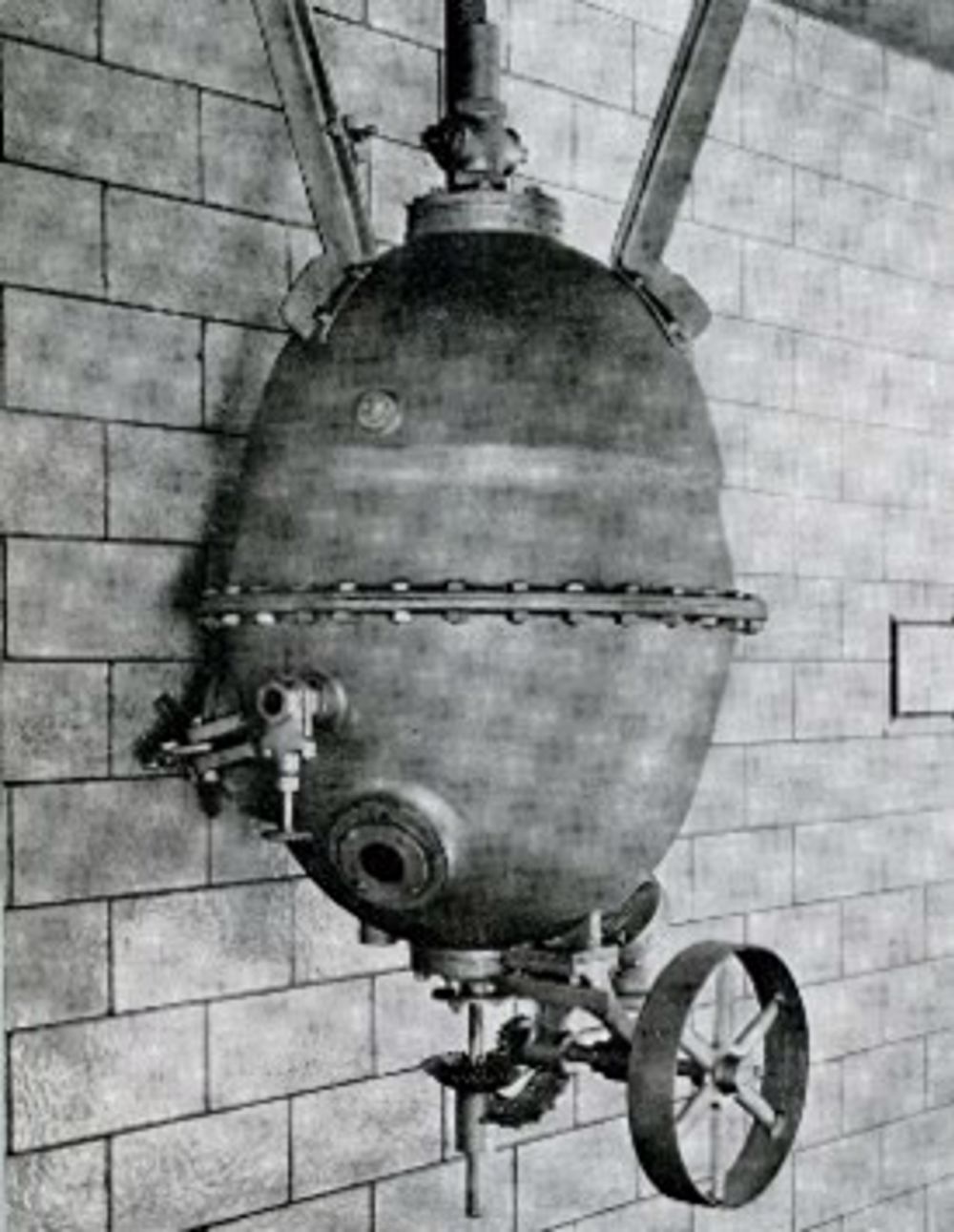
The story starts in 1909 when a patent was granted for the first synthetic plastic Bakelite, which also brought the word plastic into modern use. One of the first uses was for jewellery.
It is created from the reaction between two small molecules, formaldehyde CH2O and phenol C6H5OH which join together under heat and pressure to give a complex structure which is rigid and will not melt. Reacted inside a mould, it gives strong and hard-wearing shapes which would be expensive to manufacture by other means. It replaced ivory for billiards and snooker balls, and piano keys. Its insulating properties and non-melting behaviour made it invaluable for electrical devices. Soaked onto cotton or paper it provided the rigid insulating base in printed circuit boards from the 1920s, but mainly after the Second World War, and thus the revolution in electronics. Various other polymers have been developed since which have the property of not melting, and they are called thermosets.

In the 1920s thermoplastics (those that will soften and melt with heat) began with PVC, PolyVinylChoride, made of long chains of a simple molecule C2H3Cl vinyl chloride. This molecule is made itself ultimately from oil and salt. PVC eventually replaced rubber for electrical wiring, giving longer lasting and safer systems such as in houses and cars. It was the major material for rain and waste water piping. The amount being used has been taken as being a good indicator of the level of construction in a country. Unlike thermosets, thermoplastics can be moulded from powders, or shaped with heat from sheets or tubes, and can be welded together.
In a 1965 patent, Swedish engineer Sten Gustaf Thulin used HDPE (high density polyethelene) to produce a light strong shopping bag which could be kept folded in a pocket to avoid single-use paper bags, and thus benefit the environment. The Celloplast company was then a monopoly supplier.
In 1977 Mobil overturned the patent, and production and use of plastic shopping bags expanded massively, being often provided free for single use by supermarkets and disposed of carelessly.
Technical development in production has not been matched with development in waste disposal. Clothes being discarded after one wearing, landfill unsuitable. Multiple plastics are often found in the same small item.