The story of plastic - the 1900s to the 1970s
What is plastic? Would we all have a different answer according to the type of plastic we are most familiar with – the somewhat reviled single use bags for vegetables in the supermarket, the multitude of plastic piping attached to a hospital patient on life support, the bumper bars of modern cars? According to one source:
“Plastics are man-made materials that can be shaped in almost any form. They may be any colour of the rainbow, or as clear and colourless as crystal. Plastics may have the hardness of steel or the softness of silk. Manufacturers can shape them into long-wearing machine parts or into women’s stockings. The word ‘plastics’ comes from the Greek word ‘plastikos,’ which means ‘able to be moulded’ ”.
Plastics are polymers (long-chain molecules) or combination of such molecules with other substances to give the desired properties. The twentieth century has been the time when modern technological society came about and plastics have been at the heart of this development. Few modern necessities would be possible without them. They have displaced traditional materials like metal, wood, glass and brick in many applications. Chemical engineers have been at the centre of developing the original laboratory scale discoveries into large scale commercial processes, reducing unit cost of production and thus accelerating widespread use. This article covers the main types of plastic in commercial use up to around 1970.
The winning of a competition in New York in 1868 to find a manufacturing technique for billiard balls without needing to kill elephants to obtain tusks for the ivory traditionally used, with a material called “Celluloid” was typical of early developments in the late 19th century. These were based on chemical treatment of natural polymeric substances such as rubber and wool or cotton based cellulose. A major breakthrough came with the development of a totally synthetic plastic material by Dr Leo Baekeland in New York in 1905, formed by the reaction of phenol with formaldehyde to produce phenol formaldehyde resin. This very hard material that faithfully reproduced the contour of a mould, did not absorb water, did not melt and was a poor conductor of electricity. Commercial production of “Bakelite” started in 1910, with most products going into electrical applications. Dentures and an ‘improved’ billiard ball were amongst other products made. This is an example of a thermoset resin which once formed into its desired shape via heat and pressure can not be made pliable again by the use of heat. This is the opposite of a thermoplastic which can be softened using heat and can thus be more easily recycled.
The use of plastic materials for all kinds of applications mushroomed with thermoset resin tonnages far exceeding thermoplastics at the start of WWII, a position that had totally changed by 1950 with thermoplastics way ahead thanks to developments in the 1930’s which are described below. In the USA there was a compound growth of 20%pa from 1947 to 1950 in thermoplastics which exceeded that in thermoset resins. Plastics had been around before the war but growth had been relatively moderate. Typical uses for phenolics was in decorative light fittings, jewellery and radio cabinets. Thermoplastics before WWII comprised mainly cellulosics, with some MMA (methyl methacrylate). Wartime experiences had exposed people to many new types of products and materials developed for wartime needs, (eg. Perspex clear airplane windows and cockpits, plastic raincoats) so postwar factories changed from producing for the war effort to satisfying a new domestic market.
The development of the four major postwar thermoplastics is an interesting story:
Although it was known in the 19th century that vinyl chloride could be transformed into a solid (polymer) it was not until the late 1920’s that several companies in the USA were again working on developing polymers from vinyl compounds (i.e. monomers that have a double bond in their structure). The monomer was originally obtained by the reaction of acetylene with hydrogen chloride gas but then increasingly by thermal cracking of ethylene dichloride produced as a result of other petrochemical processes.
A major problem that had to be overcome was the rigidity of this polymer, making it difficult to convert it into useful products. It is ironic however that this rigidity led later to its major use in the construction of rain and waste water piping. The problem of rigidity was overcome by copolymerisation with vinyl acetate and the use of plasticisers. PVC became the workhorse plastic for many military uses so much so that PVC production in the USA rose very rapidly in the early 1940’s. ICI, that renowned major UK chemical producer through all of the 20th century started production of PVC compounds just before WWII using polymer imported from the USA. The company then built a vinyl monomer plant at Runcorn which was subsequently expanded when it was realised that the supply of natural rubber would likely be cut off for the remainder of the war. Distillers Chemicals Ltd (DCL) also made the monomer via a different process route. Plasticized PVC became a welcome substitute for many applications where rubber might have been used such as electrical wiring for houses, cars and aircraft. Notable is its first use in long playing records in 1948.
The first recognition of the commercial potential of this material came with a UK patent in 1911. A major problem was the reactivity of the styrene monomer which was difficult to purify and store as it had a tendency to prematurely polymerise during distillation and storage. French researchers provided an answer to this but it was an US company who were the first to build a polystyrene plant. They were helped by an eminent Russian scientist who emigrated to the USA and filed a key patent in 1927. Continuing with the multicultural theme it was scientists at Dow Chemical in the USA and I.G.Farben in Germany who started work on a commercial dehydrogenation process of ethylbenzene to produce styrene monomer around 1930. The German work proceeded faster, because of a need for the monomer to make Buna-S rubber.
Early German uses for polystyrene were in military applications, and the manufacture of articles such as combs, egg cups and drinking glasses. Post WWII it became an important new plastic for injection moulding applications and a component of adhesives, emulsions, and foams. A foamed version was patented in 1944 to give a cheap lightweight material for packing or insulation. In 1960 the first disposable foam polystyrene cups were produced. From the late 1950’s the solid clear version began to be used for disposable hypodermic syringes, which have been a major contribution to world health.
The discovery of polyethylene (often shortened to polythene) is a most remarkable story and turned out to be one of the most important inventions of the 20th century. Although some work had been carried out by a German chemist just before the turn of the 20th century it was abandoned because of the expense of the process route chosen. Both the discovery and primary development took place in the UK without other international involvement. ICI at Winnington in Cheshire were carrying out basic research into reactions at very high pressure (up to 20,000 atmospheres), when in March 1933 a test autoclave containing ethylene and benzaldehyde at 2000 atmospheres and 170degC was left overnight and on opening found to contain a white material that was later determined to be polyethylene. The experiment was repeated several times but without replicating that success and further work discontinued until 1935. It was realised that benzaldehyde did not take part in the reaction and that lower quality ethylene containing trace oxygen catalysed the polymerisation. It was said that it was fortunate that with a little less oxygen nothing would have happened and with a little more, there would have been an explosion.
ICI constructed the first commercial plant, coming on stream in 1939. Virtually all of wartime production was used in electrical insulation applications, primarily for airborne radar installations. The result was that during the early part of the war aircraft operated by Axis powers had much bulkier radar sets than Allied planes. The process was licensed by ICI and only a few years later it had become the fastest growing synthetic material in the world. The use of polyethylene was soon developed further for the manufacture of flexible piping, film for wrapping and domestic articles including washing-up bowls in a variety of colours. The latter was claimed to raise the profile and popularity of plastics in general.
In the early 1950’s academic work in Germany resulted in the discovery of a low pressure ethylene polymerisation process using a catalyst, with differing product properties to the ICI process, including a higher density. Thus this product is known as high density polyethylene (HDPE) and that from the ICI/high pressure process known as low density polyethylene (LDPE).
The polymerisation of propylene was known from the early 1930’s but it was not until 1953 that the ability to manipulate the structure of the molecule would lead to another commercially important polymer with a high melting point and good mechanical properties. Its production process is similar to that for HDPE above with similar catalysts. The first commercial plants were built in the late 1950’s and expansion in its use occurred later than with polyethylene, especially post 1970.
Others
The foregoing does not include Nylon which is a major polymer with multiple uses including synthetic textiles and is covered elsewhere. Once the basic structure of polymers was understood many different polymeric materials or polymerised mixtures were studied. Some of the more important ones are as follows:
Polyurethanes
Developed in Germany in the 1930’s from diisocyanates for protective coatings and adhesives. Polyurethane foams followed and then in the 1950’s by rigid and flexible varieties.
Polycarbonates
Produced industrially from 1960 and formed by reaction of phosgene with other chemicals and marketed successfully as light transparent sheets.
ABS(acrylonitrile, butadiene, styrene) and SAN(acrylonitrile styrene co-polymer)
These so called engineering plastics developed in the 1940’s had higher strength and/or better chemical resistance.
Silicones
Corning Glass in the USA amongst others in the 1930’s developed these materials which were water repellent, stable under heat and had good dielectric properties which made them useful when WWII started.
Polytetrafluoroethylene (PTFE)
This material was discovered by DuPont just before WWII and successfully marketed as Teflon because of its remarkable resistance to extreme temperatures, acids and friction.
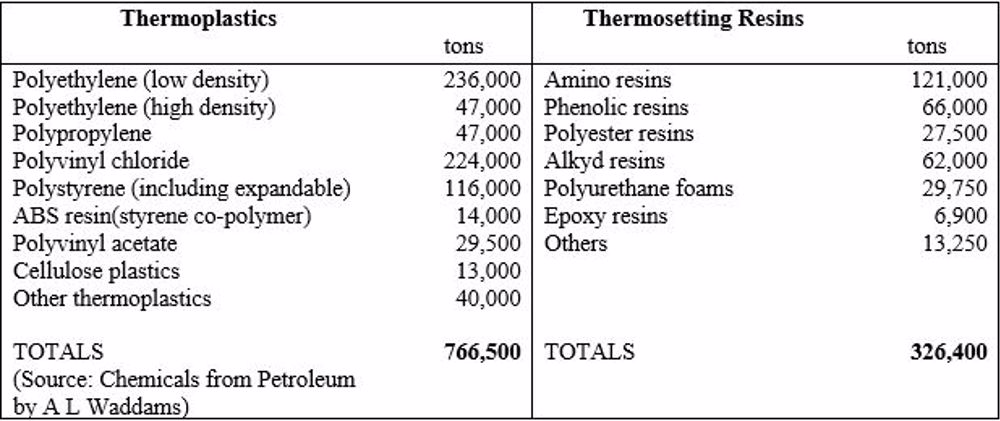
Production Tonnages
The following table shows the UK production quantities of the main thermoplastic and thermosetting plastics for 1967, a year close to 1970 and the end of this period of interest:
Amusing Reminiscences
Working on a site that made both LDPE and styrene polymers I remember the following incidents from the 1960’s:
- The LDPE polymerisation reaction taking place in very high pressure (1,000atm plus) piping safely enclosed within a blast wall would sometimes decompose with release of unreacted and reacted ethylene to atmosphere. These ‘decomps’ would be accompanied by black or white smoke but unlike the election of a new pope, there would also be a loud muffled roar as the pressure relief equipment did its job. By the end of the 60’s these events became rare.
- There would occasionally be a runaway polymerisation reaction in the polystyrene plant and the whole reactor contents would solidify. These were referred to as ‘lollipops’ as the cylindrical reactor with hemispherical bottom and stirrer axle once removed resembled the iced product of that name. Removal of the polymer was extremely difficult and defied solvents, pneumatic drills and the advice of ‘Blaster Bates’.